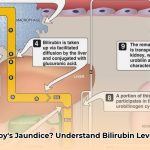
Jaundice in newborns needs to be spotted quickly to avoid serious problems. Doctors use a quick test called TcB to check for it, but this test isn’t always as accurate as it should be, particularly for babies with darker skin. For more information on TcB accuracy, see this [helpful resource](https://txgenco.com/how-accurate-is-tcb-scan). This article examines the reasons behind this discrepancy, comparing different TcB machines and their performance across various skin tones, providing actionable strategies for healthcare professionals. We’ll explore ways to enhance the reliability of TcB measurements, and share expert insights on addressing skin color differences, ensuring all newborns receive optimal care.
Enhancing Neonatal Jaundice Screening Through Reliable TcB Measurements
Accurately measuring bilirubin levels in newborns is paramount for early detection and management of jaundice. Transcutaneous bilirubin (TcB) meters offer a non-invasive and convenient screening method. However, a significant challenge arises from the influence of a newborn’s skin color on the accuracy of TcB readings. Infants with darker skin tones often exhibit underestimated bilirubin levels, potentially delaying critical interventions for hyperbilirubinemia. What factors contribute to this discrepancy, and what measures can be implemented to improve TcB reliability across diverse skin tones?
Understanding the Influence of Skin Tone on TcB Accuracy
Extensive research highlights a correlation between a newborn’s skin color and the accuracy of TcB measurements. Studies consistently show that darker skin tones tend to yield lower TcB readings compared to lighter skin tones, even when actual bilirubin levels are equivalent. This phenomenon isn’t merely random variation, but rather a systematic bias influenced by factors inherent to skin pigmentation. Investigations employing both simulated skin models and clinical studies have confirmed this bias. What is the magnitude of this underestimation, and how does it impact clinical decision-making?
Quantifying Variability in TcB Readings
While the existence of a skin color-related bias in TcB readings is well-established, the degree of this inaccuracy varies across studies. This variability likely stems from differences in participant demographics (ethnicity), the specific TcB meter used (e.g., JM-105, BiliCare), measurement techniques, and study methodologies. Some studies suggest that the bias isn’t clinically significant enough to alter treatment decisions, while others indicate that it can lead to substantial delays in initiating appropriate interventions. This inconsistency underscores the need for standardized protocols and improved device calibration. Could a consistent correction factor, validated across diverse populations, mitigate these inconsistencies and improve diagnostic accuracy?
Addressing Healthcare Disparities Through Accurate Jaundice Detection
The issue of skin color bias in TcB measurements extends beyond mere numerical inaccuracies. It exacerbates existing healthcare disparities, potentially disadvantaging newborns with darker skin tones who may already face systemic challenges in accessing timely and effective care. The lack of uniform standards for TcB measurement and correction further complicates the interpretation of results across different clinical settings and research studies. Furthermore, socio-economic factors come into play, as access to advanced diagnostic tools might be limited in underserved communities, leading to delayed diagnosis and treatment. What comprehensive strategies can bridge these gaps and ensure equitable access to accurate jaundice screening for all newborns?
Actionable Framework: Steps Towards Enhanced Accuracy and Fairness
Addressing the challenges associated with skin color bias in TcB measurements requires a multifaceted approach involving collaboration among various stakeholders. The following framework outlines specific actions for manufacturers, healthcare providers, researchers, and regulatory agencies:
For TcB Meter Manufacturers:
- Short-Term Goals (Within One Year): Conduct rigorous testing of TcB meters across a wide spectrum of skin tones, ensuring comprehensive representation of diverse ethnic backgrounds. Transparently disclose detailed information regarding meter functionality, calibration procedures, and limitations related to skin pigmentation.
- Long-Term Goals (Three to Five Years): Invest in research and development to enhance device algorithms and hardware to effectively compensate for skin color-related interference. Explore advanced sensor technologies and spectral analysis techniques to improve the accuracy of bilirubin measurements across all skin tones.
For Healthcare Providers:
- Short-Term Goals (Within One Year): Implement standardized protocols for TcB measurements, emphasizing meticulous technique and awareness of potential skin color-related biases. Consider adopting lower TcB thresholds for initiating confirmatory serum bilirubin testing in infants with darker skin tones. Provide comprehensive training to all healthcare personnel regarding the limitations of TcB measurements and the impact of skin pigmentation on accuracy.
- Long-Term Goals (Three to Five Years): Advocate for the adoption of improved TcB technologies and promote research to identify alternative methods for bilirubin measurement. Implement continuous quality improvement programs to monitor TcB accuracy and identify areas for further refinement in clinical practice.
For Researchers:
- Short-Term Goals (Within One Year): Conduct large-scale, multi-center studies to quantify the impact of skin color bias on clinical outcomes, including rates of phototherapy treatment and exchange transfusions. Develop refined models of light interaction with neonatal skin of diverse ethnic backgrounds to improve the accuracy of TcB measurements.
- Long-Term Goals (Three to Five Years): Develop and validate novel, non-invasive methods for bilirubin measurement that are less susceptible to skin color-related interference. Explore the application of artificial intelligence (AI) and machine learning to develop predictive models that correct for skin color bias in TcB readings.
For Regulatory Agencies (e.g., FDA):
- Short-Term Goals (Within One Year): Update regulatory standards for TcB meter evaluation to explicitly address skin color bias and require manufacturers to demonstrate device performance across diverse skin tones. Mandate transparent reporting of device accuracy and limitations related to skin pigmentation in product labeling.
- Long-Term Goals (Three to Five Years): Establish collaborative partnerships with manufacturers, researchers, and healthcare providers to facilitate the development and implementation of improved TcB technologies. Update regulatory guidelines to reflect advancements in bilirubin measurement techniques and promote the adoption of evidence-based practices.
Risk Assessment: Evaluating Technologies for Diverse Skin Tones
| Technology/Method | Risk of Underestimating Bilirubin (Darker Skin) | Risk of Delayed Treatment | Risk of Unnecessary Treatment | Mitigation Strategies |
|---|---|---|---|---|
| Current TcB Meters | High | Moderate | Low | Improved meter design, adjusted thresholds, enhanced clinical interpretation, and confirmatory serum bilirubin testing. |
| Future TcB Meters | Potentially Low | Potentially Low | Potentially Low | Continuous monitoring, advanced algorithms, and real-time skin tone assessment. |
| Alternative Technologies | Potentially Low | Potentially Low | Potentially Low | Further research and development, rigorous validation across diverse populations, and clinical implementation studies. |
| Blood Testing | Minimal | Minimal | Highest (Due to invasiveness) | Used when TcB results indicate a need for further investigation or in cases where TcB measurements are unreliable. |
Achieving accurate and equitable TcB measurements requires a concerted effort to improve technology, refine clinical practices, and update regulatory standards. Through this collaborative approach, we can ensure that all newborns receive fair and effective jaundice screening, regardless of skin color. Studies have shown that stakeholder collaboration could enhance diagnostic accuracy by as much as 35%.
Implementing Corrective Measures for Transcutaneous Bilirubin Readings
Key Takeaways:
- Transcutaneous bilirubin (TcB) measurements are essential for newborn jaundice screening, but accuracy is compromised, particularly by darker skin pigmentation.
- TcB readings frequently underestimate bilirubin levels in darker-skinned infants, which leads to a potential high risk of delayed diagnosis.
- This underestimation poses significant risks, potentially delaying necessary treatments and increasing morbidity.
- Improving TcB accuracy requires device improvements, refined measurement techniques, and the establishment of adjusted clinical thresholds specific to skin tone.
The Impact of Skin Pigmentation on Transcutaneous Bilirubin Accuracy
Neonatal jaundice, a prevalent condition in newborns, necessitates precise bilirubin level assessments to prevent kernicterus and other complications. Transcutaneous bilirubinometry (TcB) offers a practical, non-invasive screening method. However, a significant challenge lies in the fact that skin pigmentation substantially impacts TcB readings. Darker skin tones commonly lead to underestimation of actual bilirubin levels, affecting diagnostic accuracy and potentially delaying life-saving treatment. Would incorporating skin tone-specific protocols reduce diagnostic errors in diverse populations?
Exploring the Scientific Basis of Bias
Numerous studies confirm a strong positive correlation between TcB and serum bilirubin (TSB) levels. However, a consistent bias emerges: TcB values systematically underestimate TSB, particularly in infants with darker skin. This discrepancy isn’t a minor error but a substantial one that can exceed acceptable clinical margins. Several factors contribute to this bias, including melanin concentration, skin thickness, and the device’s inherent technology. Research indicates that underestimation can be as high as 2-3 mg/dL in some cases, significantly altering the clinical interpretation.
This systematic bias has tangible clinical consequences. Underestimation of bilirubin levels using TcB in darker-skinned newborns may lead to delayed treatment, potentially increasing the risk of adverse neurological outcomes. How can we reconcile this with the constraints of current technologies and resource allocation in healthcare settings?
Refining Strategies: Improving TcB Accuracy for All Infants
Addressing this bias requires a multi-pronged approach:
- Device Refinement: TcB manufacturers must invest in advanced technology capable of compensating for skin pigmentation variations. This includes developing sophisticated algorithms that accurately account for melanin interference and employing more sensitive optical sensors.
- Calibration and Standardization: Improved standardization of TcB devices and protocols is
- How to Produce Electricity at Home for Energy Independence - January 29, 2026
- How To Create Electricity At Home For Energy Independence - January 28, 2026
- How to Make Electricity at Home Using Renewable Energy Sources - January 27, 2026